Con Lăn
Heat exchangers
Have you ever watched wisps of smoke drifting from smokestacks and wondered how much energy they're uselessly pumping into the air? Maybe less than you might think! Saving energy is a huge and costly problem for factory bosses and it's one reason they often install devices called heat exchangers to salvage as much heat as possible from waste gases. Heat exchangers have lots of other familiar uses too. Engines in cars, ships, and planes use heat exchangers to work more efficiently, gas boilers use them to heat up hot water, and if you have a refrigerator or an air-conditioner in your home, those are using heat exchangers too. So what exactly are heat exchangers and how do they work? Let's take a closer look!
Photo: Heat exchangers are used in many engines and machines to improve their efficiency. Photo by Michael J. Lieberknecht courtesy of US Navy.
Contents
What is a heat exchanger?
Suppose you have a gas central heating furnace (boiler) that heats hot-water radiators in various rooms in your home. It works by burning natural gas, making a line or grid of hot gas jets that fire upward over water flowing through a network of pipes. As the water pumps through the pipes, it absorbs the heat energy and heats up. This arrangement is what we mean by a heat exchanger: the gas jets cool down and the water heats up.
A heat exchanger is a device that allows heat from a fluid (a liquid or a gas) to pass to a second fluid (another liquid or gas) without the two fluids having to mix together or come into direct contact. If that's not completely clear, consider this. In theory, we could get the heat from the gas jets just by throwing cold water onto them, but then the flames would go out! The essential principle of a heat exchanger is that it transfers the heat without transferring the fluid that carries the heat.

Photo: How a simple heat exchanger works. A hot fluid (shown in red) flows through a tube coiled inside a larger shell through which another, colder fluid (shown in blue) is running in the opposite direction. Heat is exchanged by the fluids: the hot fluid cools down and the cold fluid warms up, without them actually coming into contact and mixing. This is a simplified example of a shell and tube exchanger: generally, heat exchangers of this design have many thin tubes running through a large shell.
You can see heat exchangers in all kinds of places, usually working to heat or cool buildings or helping engines and machines to work more efficiently. Refrigerators and air-conditioners, for example, use heat exchangers in the opposite way from central heating systems: they remove heat from a compartment or room where it's not wanted and pump it away in a fluid to some other place where it can be dumped out of the way. The cooling fluid is completely sealed inside a network of pipes, so it never actually comes into contact with the air: it takes heat energy from the air inside and dumps it in the air outside, but it never mixes directly with that air.

Photo: A heat pump extracts heat from a natural geothermal hot spring, used to heat buildings at Hot Springs Lodge and Pool in Glenwood Springs, Colorado. The exchanger is the algae-covered plate full of copper tubes in the center of the water. Photo by Warren Gretz courtesy of US DOE/NREL (Department of Energy/National Renewable Energy Laboratory).
In power plants or engines, exhaust gases often contain heat that's heading uselessly away into the open air. That's a waste of energy and something a heat exchanger can certainly reduce (though not eliminate entirely—some heat is always going to be lost). The way to solve this problem is with heat exchangers positioned inside the exhaust tail pipes or smokestacks. As the hot exhaust gases drift upward, they brush past copper fins with water flowing through them. The water carries the heat away, back into the plant. There, it might be recycled directly, maybe warming the cold gases that feed into the engine or furnace, saving the energy that would otherwise be needed to heat them up. Or it could be put to some other good use, for example, heating an office near the smokestack.
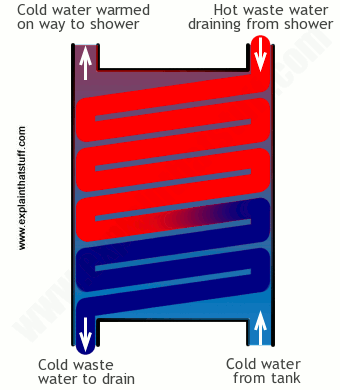
Photo: How a shower waste-water heat exchanger works. Hot outgoing waste-water warms incoming cold water, reducing the energy you need to get the water hot and making the whole thing more efficient.
In buses, fluid used to cool down the diesel engine is often passed through a heat exchanger and the heat it reclaims is used to warm cold air from outside that is pumped up from the floor of the passenger compartment. That saves the need for having additional, wasteful electric heaters inside the bus. A car radiator is another kind of heat exchanger. Water that cools the engine flows through the radiator, which has lots of parallel, aluminum fins open to the air. As the car drives along, cold air blowing past the radiator removes some of the heat, cooling the water and heating the air and keeping the engine working efficiently. The radiator's waste heat is used to heat the passenger compartment, just like on a bus.
If you have an energy-efficient shower, it might have a heat exchanger installed in the wastewater outlet. As the water drips past your body and down the plug, it runs through the copper coils of a heat exchanger. Meanwhile, cold water that's feeding into the shower to be heated pumps up past the same coils, not mixing with the dirty water but picking up some of its waste heat and warming slightly—so the shower doesn't need to heat it so much.
Types of heat exchangers
All heat exchangers do the same job—passing heat from one fluid to another—but they work in many different ways. The two most common kinds of heat exchanger are the shell-and-tube and plate/fin. In shell and tube heat exchangers, one fluid flows through a set of metal tubes while the second fluid passes through a sealed shell that surrounds them. That's the design shown in our diagram up above. The two fluids can flow in the same direction (known as parallel flow), in opposite directions (counterflow or counter-current), or at right angles (cross flow). Boilers in steam locomotives work this way.

Animation: How a counterflow shell and tube heat exchanger works. 1) The exchanger consists of a large outer shell (light gray) with tubes running through it (dark gray). 2) A hot fluid enters the tubes from the top left and exits from the bottom right. 3) A cooler fluid passes the opposite way through the shell, entering at the bottom right and exiting at the top left. Baffles (black lines), which deflect the fluid flowing through the shell, help to ensure that it passes its heat evenly to all the tubes.

Artwork: A simple example of a plate/fin heat exchanger. This is a cross-flow design with the two fluids flowing past one another at right angles.
The photos below show some examples of real heat exchangers:


Photo: Two types of heat exchanger. 1) A shell and tube exchanger from the Savannah River nuclear plant in South Carolina, United States. There are lots of tubes in this one and they're easy to see. Photo by courtesy of US Department of Energy (DOE). 2) The plate and fin heat exchanger from inside a gas central heating boiler/furnace.
Heat exchangers used to minimize heat losses from buildings, engines, and machines are sometimes called recuperators or regenerators. These are two quite different things. A recuperator is typically used to capture heat that would otherwise be lost, for example, as stuffy air is ventilated from a building: cold, incoming fluid is channeled in the opposite direction to warm, outgoing fluid to minimize the heat loss. The two fluids flow through separate channels, remain separate, and do not mix. Since incoming and outgoing fluids move in opposite directions, recuperators are examples of counterflow heat exchangers. The heat exchanger in a heat-recovery ventilation (HRV) system is an everyday example of a recuperator. Warm stuffy air flowing out of a building gives up its heat to cold, fresh air flowing in from outside, so the building gets properly ventilated without losing all its heat.

Photo: A shell and tube heat exchanger photographed at the Idaho National Engineering Laboratory Test Reactor in 1956. In this unit, a coolant fluid enters the exchanger at 26°C (78°F) and leaves at 43°C (110°F), taking heat from water that enters at 59°C (137.5°F) and leaves at 43°C (110°F). Photo by R.G. Larsen courtesy of Library of Congress, Prints & Photographs Division, HAER, Reproduction number HAER ID-33-G-317..
A regenerator is similar, but the incoming and outgoing fluids move through the same channel in opposite directions and at different times. So the warm fluid will flow out through the regenerator, giving up some of its heat on the way. Later, the cold fluid will flow in through the same channel, back through the regenerator, picking up some of the heat stored there. A regenerator is one of the key parts in a highly efficient form of power called a Stirling engine, in which a piston pushes trapped gas back and forth between a heat source (such as a fire) and a place where the heat is lost ("a sink"). The regenerator reduces the heat that would otherwise be lost as the engine cycles back and forth.
What are the best materials for a heat exchanger?

Photo: Plastics are perfectly suitable materials for low-temperature heat exchangers.
You might think heat exchangers would always need to be made of metals, which quickly absorb and conduct heat—and many of them are. But heat exchangers can also be made of ceramics, composites (based on either metals or ceramics), and even plastics (polymers).
All these materials have their advantages. Ceramics are a particularly good choice for the kind of high-temperature applications (over 1000°C or 2000°F) that would melt metals like copper, iron, and steel, though they're also popular for use with corrosive and abrasive fluids at either high or low temperatures. Plastics generally weigh and cost less than metals, resist corrosion and fouling, and can be engineered to have good thermal conductivity, though they tend to be mechanically weak and may degrade over time. Although not generally suitable for high-temperature applications, plastic exchangers could be a good choice for something like a swimming pool or shower, operating at everyday, room-temperatures. Composite heat exchangers combine the best features of their parent materials—say, the high thermal conductivity of a metal with the reduced weight and better corrosion resistance of a plastic.
In the future, it's distinctly possible we'll be using even more interesting materials in heat sinks. Carbon nanotubes, for example (thin hexagonal sheets of carbon wrapped around to make "pipes"), have amazing heat conducting properties and are already being used in heat sinks (heat removal devices used mainly in electronics). Lots of research is currently being done to see how they can be deployed in heat exchangers.




